Mechanistic Studies on the Mukaiyama Epoxidation
Bastienne Wentzel
20 april 2004, Wetenschappelijke publicaties
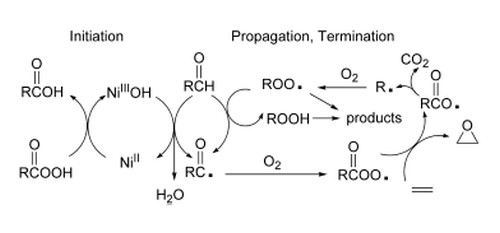
A detailed mechanistic study on the Mukaiyama epoxidation of limonene with dioxygen as oxidant, bis(acetylacetonato)nickel(II) as catalyst, and an aldehyde as co-reagent is reported.
Abstract
All major products of the reaction have been quantitatively identified, both with isobutyraldehyde and 2-methylundecanal as co-reacting aldehydes.
Limonene epoxide is formed in good yield. The main products evolving from the aldehyde are carboxylic acid, CO2, CO, and lower molecular weight ketone and alcohol (K + A).
A mechanism is proposed in which an acylperoxy radical formed by the autoxidation of the aldehyde is the epoxidizing species. The observation of carbon dioxide and (K + A) in a 1:1 molar ratio supports this mechanism. CO2 and (K + A) are formed in molar amounts of 50-60% with respect to the amount of epoxide produced, indicating that epoxidation takes place not only via acylperoxy radicals but also via a peracid route.
Cyclohexene epoxidation was also investigated with a number of different metal complexes as catalysts. Cyclohexene is very sensitive for allylic oxidation, which provides information about the action of the catalyst, e.g., metals that form strongly oxidizing stable high-valence complexes are more likely to induce allylic oxidation.
Color changes in the reaction mixture indicate the presence of such high-valence species. In the case of nickel, it was found that low-valence compounds predominate during the reaction, which is in line with the fact that this metal displays the highest selectivity for epoxide.
A mechanism that accounts for the observations is presented.
J. Org. Chem., 2004, 69 (10), 3453-3464
Bastienne B. Wentzel, Paul L. Alsters, Martinus C. Feiters, and Roeland J. M. Nolte
