Abstract
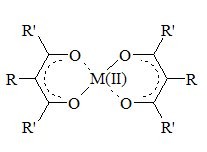
The scope, mechanism and kinetics were studied of the aerobic epoxidation of alkenes with an aldehyde and substituted beta-diketonate-transition metal complexes as catalysts. Diketonate complexes of nickel(II) proved to be among the best catalysts for this reaction. The epoxidation is not dependent on substrate concentration and is first order in aldehyde, catalyst and oxygen partial pressure. It was shown by reactivity studies and EPR experiments that the reaction is radical in nature. Additional evidence for this radical nature was obtained from stereochemical investigations. The metal catalyst is not only an efficient initiator of the reaction, but is also believed to enhance the reactivity of intermediate species in the oxidation process by allowing these to coordinate to the metal center. A mechanism is proposed for the catalytic reaction.
J. Chem. Soc., Dalton Trans. 1998, 2241-2246
Bastienne B. Wentzel, Patricia A. Gosling, Martin C. Feiters and Roeland J. M. Nolte